🔢 Data Splits and Acquisition Details 🔢
📗 Open Training Set (3095 cases) 📗
This dataset is made available for all participants and researchers, and consists of fully anonymized endoscopic images. It is made publicly under a non-commercial CC-BY-NC-SA license. The dataset comprises 2937 normal, non-dysplatic cases and 158 neoplasia cases, and will be the largest publicly available dataset focussed on Barret's esophagus in endoscopic images to date.
All training images were acquired using the Exera III system (Olympus Corp., Tokyo, Japan). The training data was collected retrospectively via an automated query from EndoBase, designed to broadly retrieve all stored images from the system. From this dataset, Barrett’s esophagus-related images were manually identified and extracted. No standardized imaging protocol was in place during the original acquisition. As a result, imaging parameters, techniques, and device settings may vary, depending on local clinical practices and individual operator preferences. The data was sourced from routine endoscopic surveillance procedures at two medical centers in the Netherlands. Data collection occurred as part of standard clinical workflows, without the involvement of a specific expert group. Therefore, detailed information regarding the experience level of the medical professionals involved is not available.
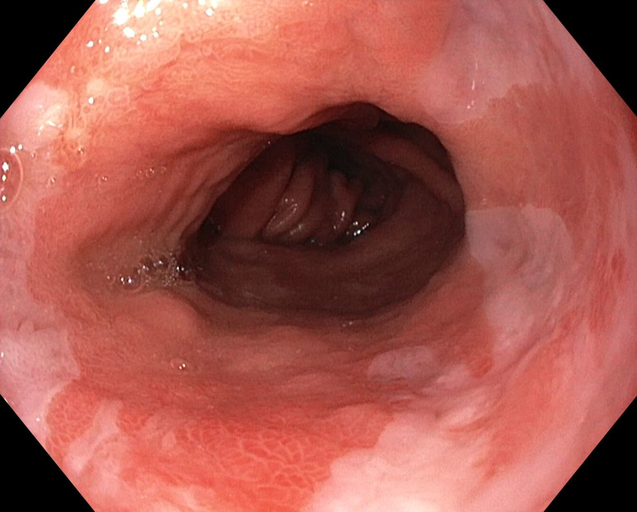
Neoplastic Barrett's Esophagus
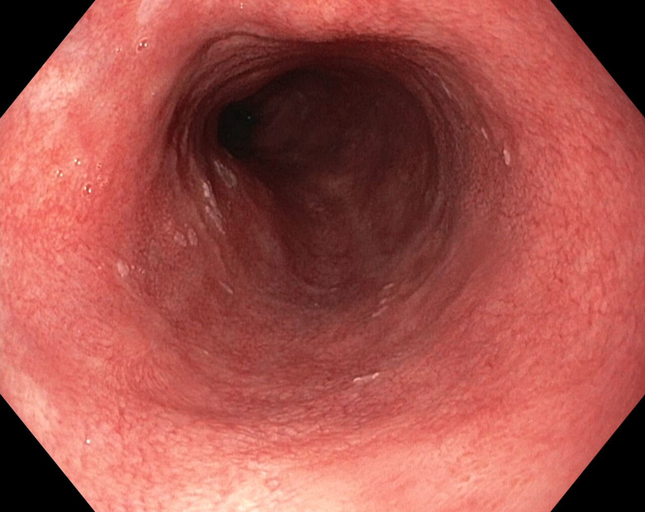
Non-Dysplastic Barrett's Esophagus
📕 Closed Validation and Testing Cohort 📕
This dataset will remain private and is solely available to the challenge organizers. It will be used for the evaluation of the algorithms during the Open Development Phase (Validation Cohort) and Closed Testing Phase (Testing Cohort).
All the validation and test images were acquired using the Exera III system (Olympus Corp., Tokyo, Japan). The dataset was constructed using both retrospective and prospective data collection methods. The restrospective images were retrieved from EndoBase archives using pre-identified patient lists, each associated with corresponding pathology reports. All retrieved images were manually reviewed to assess the presence or absence of abnormalities. The prospective images were obtained through a standardized prospective acquisition protocol, carried out by expert endoscopists specializing in Barrett’s esophagus screening. The Validation and Testing cohort includes cases from 12 medical centers participating in the BONSAI consortium, as described in Fockens et al. (2023).